Our lab studies protein quality control and protein homeostasis. We are very interested in how ubiquitin and ubiquitin-related processes function in the cell. We are focused on proteins associated with neurodegenerative and neuromuscular diseases such as amyotrophic lateral sclerosis (ALS).
We currently focus on examining the structure, dynamics, and function of ubiquilins, shuttle proteins that are involved in proteasome degradation and autophagy. Ubiquilins are also implicated in neurological disorders. For example, both wild-type and mutant UBQLN2 protein is found in protein-containing inclusions of degenerated motor neurons in ALS patients and mouse models.
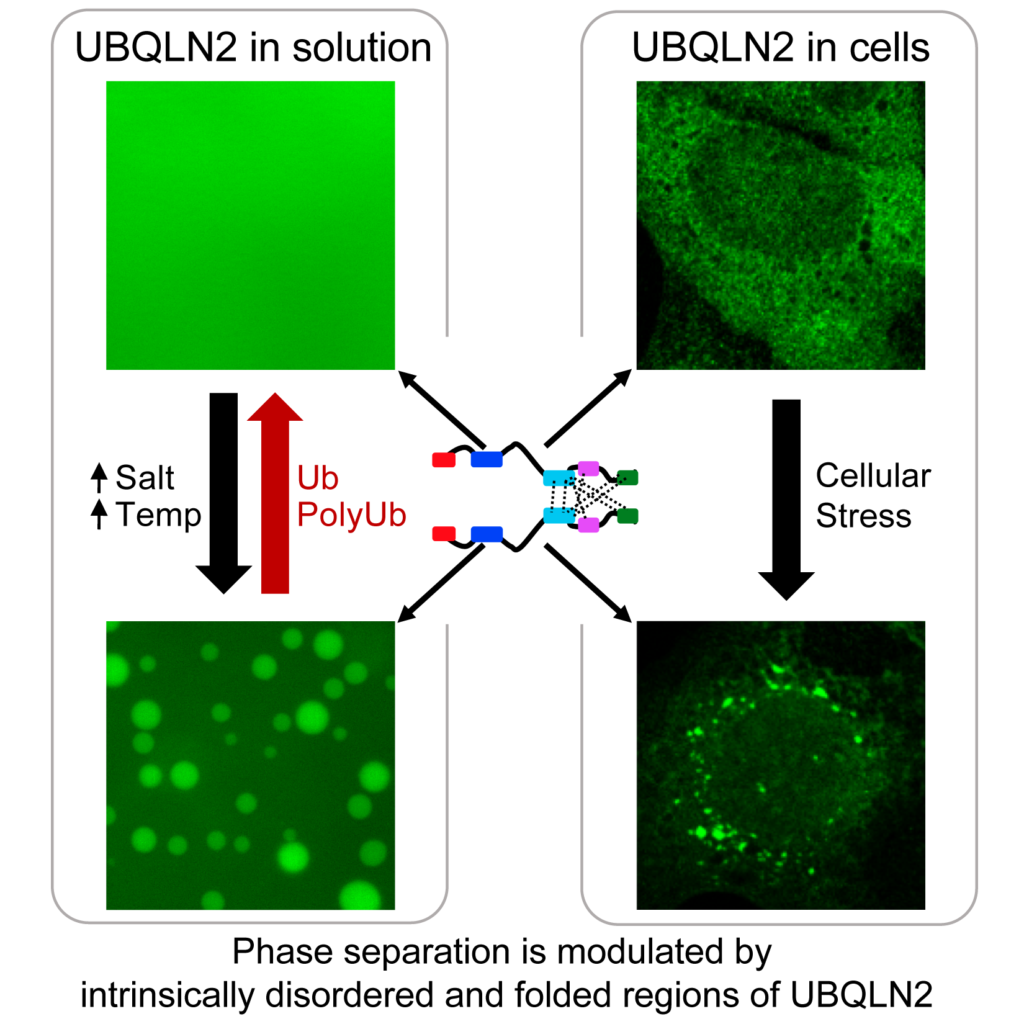
We recently determined that UBQLN2 undergoes liquid-liquid phase separation (LLPS), a process by which proteins self-associate into biomolecular condensates. We showed that UBQLN2 is recruited to stress granules, a type of membraneless organelle that is hypothesized to form via phase separation of its components including proteins and RNA. Our lab is thus currently focused on understanding the molecular mechanisms governing LLPS of ubiquilins, and how LLPS is disrupted in disease. To address these important and broad questions, we use a variety of interdisciplinary, experimental and computational techniques, including biomolecular NMR, microscopy, small angle scattering, mass spectrometry, and simulations. We are also expanding into cell biology approaches to better understand the function of these proteins in cells. We have built exciting collaborations with multiple labs in the Syracuse area and across the U.S. to help us achieve our goals. See our research pages for more information.
In addition, we are interested in the effects of post-translational modifications such as ubiquitination on proteins associated with neurological disorders.
If you are interested in joining our research program, please contact Prof. Castañeda! Our lab is always looking for motivated, excited Chemistry and Biology graduate and undergraduate students. Interested undergraduate freshmen and sophomores are especially encouraged to apply.