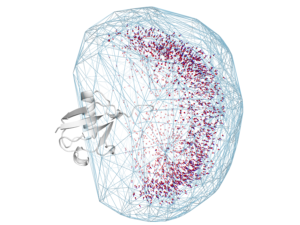
Over 70% of eukaryotic proteins are multidomain proteins (consisting of multiple units connected by linkers). For example, RNA-binding proteins often consist of multiple domains separated by linkers. We are interested in determining how these linkers contribute to protein function.
A first goal of this project is to determine conformational ensembles of two-domain proteins to better understand how these linkers contribute to multidomain protein flexibility. We currently are using non-canonical polyubiquitin chains, and hope to move onto other multi-domain protein systems.
Another common aspect of these linkers is that they are generally accessible for post-translational modifications. We aim to determine how PTMs on interdomain linkers affect their flexibility and function.